FDA Approves Long-Acting Monoclonal Antibody Infusion Every 3 Months to Protect Immunocompromised From COVID-19
Pemvibart Late Addition to Armamentarium after Most Have Natural Immunity by 2024
By Peter A. McCullough, MD, MPH
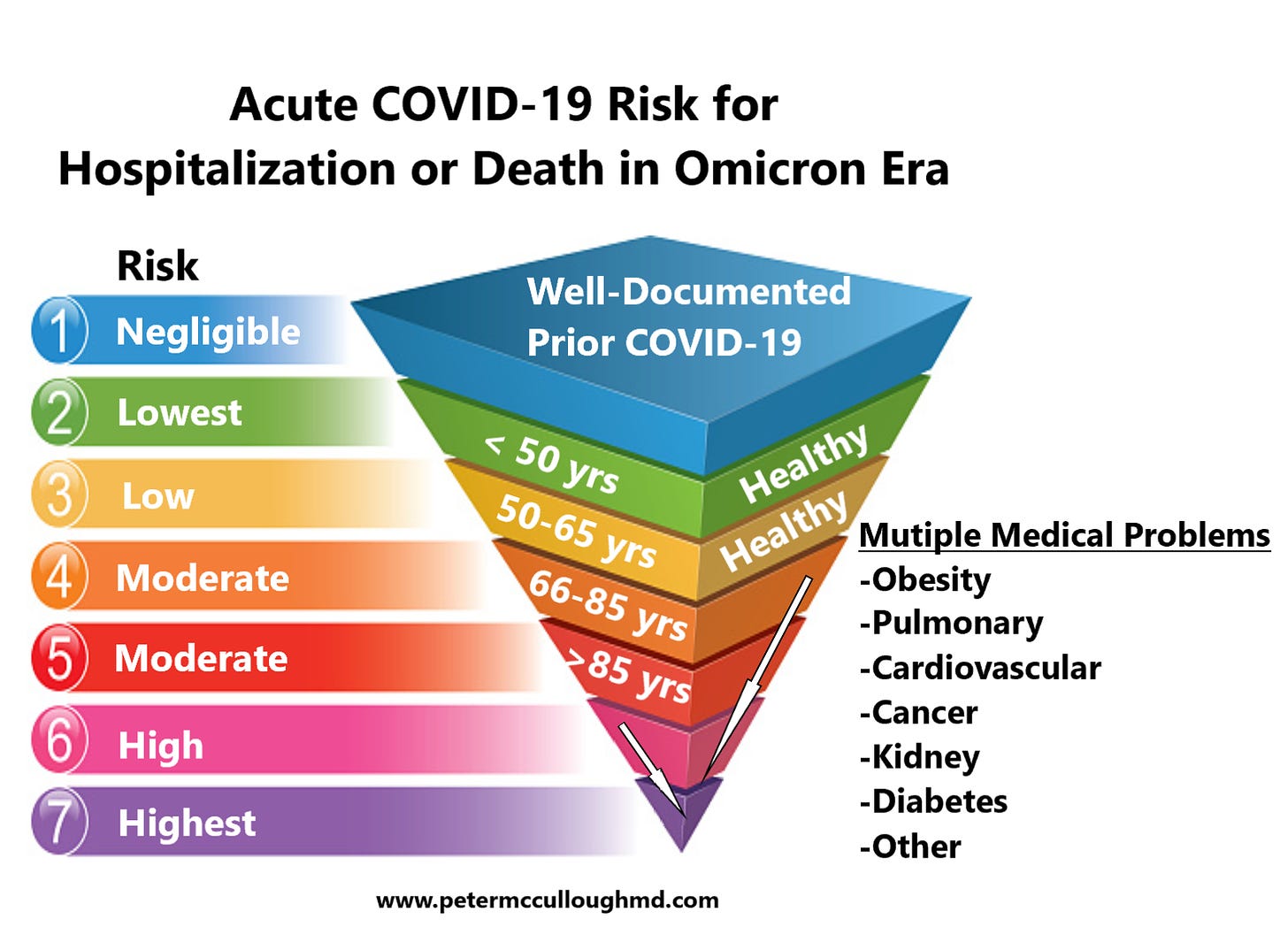
Most Americans including the immunocompromised have had at least one episode of acute SARS-CoV-2 infection, and therefore have negligible risk of hospitalization or death on a subsequent infection. However, those who are very vulnerable and have not encountered COVID-19, could benefit from a new, longer-acting monoclonal antibody recently approved by the US FDA.
The FDA has authorized pemivibart (Pemgarda; Invivyd) for emergency use in the prevention—or pre-exposure prophylaxis (PREP)—of COVID-19 in adults and adolescents who are 12 years of age and older who either have moderate to severe immune compromise because of medical conditions or receive certain immunosuppressive medications. It is an alternative to the failed COVID-19 vaccines.
Pemivibart, which was formerly known as VYD222, is a half-life extended monoclonal antibody (mAb). The EUA for pemivibart is based on data from the CANOPY (NCT06039449) clinical trial, which demonstrated positive results. The CANOPY trial is an ongoing phase 3 trial that enrolled patients to receive either pemivibart or placebo. Cohort A was an open-label, single-arm trial which enrolled patients aged 18 years or older with moderate to severe immune compromise (n = 306) facing relevant SARS-CoV-2 variants (eg, JN.1) in the analyses of neutralizing titers and the primary immunobridging endpoint for cohort A. The results indicated an expected 70% relative risk reduction in the development of symptomatic COVID-19 between treatment and placebo arms. With additional agents in the McCullough Protocol, even the most severely immunocompromised should get through the illness without risks for hospitalization or death. The most serious safety event was anaphylaxis occuring in 4 participants (0.6%). Of these patients, 2 experienced anaphylaxis during the first infusion of pemivibart, and the remaining 2 during the second infusion.
I will consider patients with oxygen-dependent severe lung disease who are immunocompromised without natural immunity for these infusions. I anticipate cases such as these will be very rare as we progress through the pandemic.
Please subscribe to Courageous Discourse as a paying or founder member so we can continue to bring you the truth.
Peter A. McCullough, MD, MPH
President, McCullough Foundation






Monoclonal antibodies should be used as a potential life saving emergency intervention similar to using antivenom in a person bitten by a poisonous snake. Antibodies from recently convalesced individuals can effectively be used AFTER exposure, but using mass produced stored antibodies BEFORE exposure as a prophylactic thinking this will prevent the infection from happening is a bad idea. The problem is, the cells of our bodies are continuously mutating the genetic code of the virus. That's why there are so many clades and variants. So, you end up loading these monoclonal antibody recipients with something that might be miss-matched. This primes them for antibody dependent enhancement of infection, meaning the antibodies will signal immune cells to replicate the virus instead of destroying it. It also primes them for antibody dependent enhancement of disease, meaning it can kick off the complement cascade and worsen symptoms after exposure. Again, monoclonal antibodies should be used as a treatment, not a prophylactic.
And so approval comes now, at a time when it cannot undermine or interfere with the mass injection campaign.