Mixed Initial and Revaccination with Bacille Calmette-Guérin (BCG) Vaccine for COVID-19
Poorly Designed Randomized Trial, Unimpressive Results in Low-Risk Healthcare Workers
By Peter A. McCullough, MD, MPH
Early observations in regions where the antituberculosis Bacille Calmette-Guérin (BCG) Vaccine was used indicated lower rates of COVID-19 and mortality. The hypothesis was that general activation of cellular elements of the immune system would protect patients against severe disease from SARS-CoV-2.
Gong, summarized > 50 studies described the clinical trials through mid 2022: “As early as July 17, 2020, ICMR-National Institute for Research in Tuberculosis conducted a phase 3 clinical trial to evaluate the effectiveness of the BCG vaccine in decreasing morbidity and mortality of elderly individuals with COVID-19 in India (NCT04475302). The preliminary results observed the proliferation of plasma-like dendritic cells and myeloid dendritic cells and increased levels of IFN-λ1 (IL-29), IFN-λ2 (IL-28a), and IFN-λ3 (IL-28b) and decreased levels of IFN-α and IFN-βin plasma induced by BCG vaccination [141]. These results provide indirect evidence for BCG vaccination to fight SARS-CoV-2 infection by enhancing heterologous immunity.
In a double-blind, randomized phase III ACTIVATE-2 study (NCT04414267), participants were randomized to receive either BCG revaccination or placebo to evaluate whether BCG vaccination can prevent COVID-19 infection in the elderly population [142]. During the 6-month observation, BCG revaccination reduced the risk of COVID-19 clinical and microbiological diagnosis by 68%, suggesting its protective effect in the elderly against COVID-19. Interestingly, three months after BCG vaccination, anti-SARS-CoV-2 antibodies that were induced in mild or asymptomatic infection were detected in more BCG-vaccinated volunteers (4.7%) than the placebo (1.3%), indicating that BCG revaccination may improve the low antibody responses and enhance its protection against COVID-19 infection in mild or asymptomatic patients [142]. This preprinted study provided substantial evidence of the protective efficacy of BCG on COVID-19 prevention. However, one drawback of the study is the small sample size.
Another similar study recruited HCWs who had the BCG vaccine administered at birth in the United Arab Emirates and gave them a booster BCG vaccination or not [143]. There were significant differences in the rate of COVID-19 infection between the booster BCG vaccination group and the BCG unvaccinated group (zero (0/71) vs. 8.6% (18/209), P = 0.004), demonstrating the potential value of a booster BCG vaccination in preventing COVID-19 infectionCOVID-19.
Except for the prophylactic protection effects, the potential therapeutic effect of BCG for COVID-19 control was also evaluated by a Phase II clinical trial. Sixty COVID-19 patients with pneumonia requiring oxygen therapy were recruited and were given an intradermal injection of BCG or normal saline (control group) [144]. Compared with the control group, the BCG group was found to have significantly faster resolution of COVID-19 associated hypoxia, significantly radiological improvement from day 7–15, and a substantially higher percentage of patients with favourable outcomes. These findings suggest that BCG may be a safe and cost-effective treatment that can be used as a standard for treating patients with moderate COVID-19 by reducing the need for oxygen replenishment beds and disease burden in resource-poor countries.
After the vaccines against SARS-CoV-2 were available, the enhancement effect of BCG on the vaccines was also evaluated. A study exploring the effect of revaccination with BCG on the response to a subsequent anti-SARS-CoV-2 vaccine in persons occupationally exposed to COVID-19 patients found that prior BCG vaccination enhanced the increase of serum cytokine concentrations and higher neutralizing antibody titers, indicating the synergistic effects of BCG on subsequent vaccination against SARS-CoV-2 [145].
A most recent multicenter, randomized, double-blind, and placebo-controlled study (Register No. of NCT04648800 at ClinicalTrials.gov) on HCWs in Poland didn’t acquire similar results [146]. The participants in the study with a negative tuberculin test were randomized (1:1), received either the BCG- or placebo vaccine, and then subjected to observation for COVID-19 symptoms occurrence. The BCG vaccination, the tuberculin test result, and the number of scars were not observed to correlate with the suspected COVID-19 incident rate in statistical analysis. However, before the trial, all the participants had previously received at least two doses of the BCG vaccine due to the vaccination policy of Poland, which may decrease the variation between the BCG revaccination group and placebo group and is a significant limitation of this study [146]. In this sense, a control group with never-vaccinated participants may lead to a different result.”
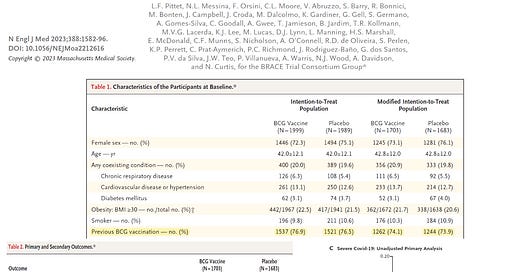
With is background, the BRACE trial recently reported neutral results in the New England Journal of Medicine. Careful inspection of the study indicated that 75% of the healthcare workers who enrolled as subjects already had the BCG shot, so both the active BCG and placebo groups had only 25% of unvaccinated subjects. The overall rate of hospitalization or death was 9/3386 (0.2%). Thus the most important finding is irrespective of any vaccine strategy, healthcare workers are at negligible risk of severe outcomes with COVID-19, and thus, any vaccine would have an insignificant theoretical benefit.
Keep reading with a 7-day free trial
Subscribe to FOCAL POINTS (Courageous Discourse) to keep reading this post and get 7 days of free access to the full post archives.