Renal Denervation Approved for Treatment of Hypertension
Devices from Recor and Medtronic Released onto US Market
By Peter A. McCullough, MD, MPH
For patients who are seeking relief from prescription pharmaceuticals, a new interventional procedure has been recently FDA approved in November, 2023, that may warrant consideration.
Renal artery denervation is a minimally invasive procedure performed by an interventional cardiologist or radiologist guiding a catheter into the renal arteries. From there, pulses of ultrasound or radiofrequency energy are used to damage some of the sympathetic nerves in the arteries. The goal of the procedure is to lower a patient’s blood pressure by reducing overactivity in those nerves.
MedTechDive gave additional granularity:
Recor’s Paradise system, which the FDA approved in early November, uses ultrasound for ablation, while Medtronic’s Symplicity Spyral system, approved later that month, uses radiofrequency energy. Both devices are labeled as adjunctive treatments for when medications and lifestyle changes aren’t enough to control patients’ blood pressure. Lara Barghout, Recor’s CEO, described it as a “wide label,” in the sense that it didn’t specify patients must have uncontrolled or resistant hypertension.
An FDA advisory panel in August recommended that the FDA approve Recor’s device but voted against approval of Medtronic’s device. For Recor’s Paradise system, panelists voted unanimously in favor of the device’s safety and 10-2 in favor of its risk-benefit profile, raising questions about the long-term durability of the treatment.
For Medtronic’s Symplicity Spyral system, the panelists voted unanimously for safety but found the benefits did not outweigh the risks by a 6-7 vote. They noted that a recent study of the device missed its primary endpoint, while an earlier study only showed a small benefit.
Keith Allen, surgical director of the structural heart program at Saint Luke’s Mid America Heart Institute in Kansas City, said he voted no on both devices in the panel.
“I didn’t think either device from Recor or Medtronic met the level that warranted approval,” he said in an interview. “The data, in my opinion, is not very robust.”
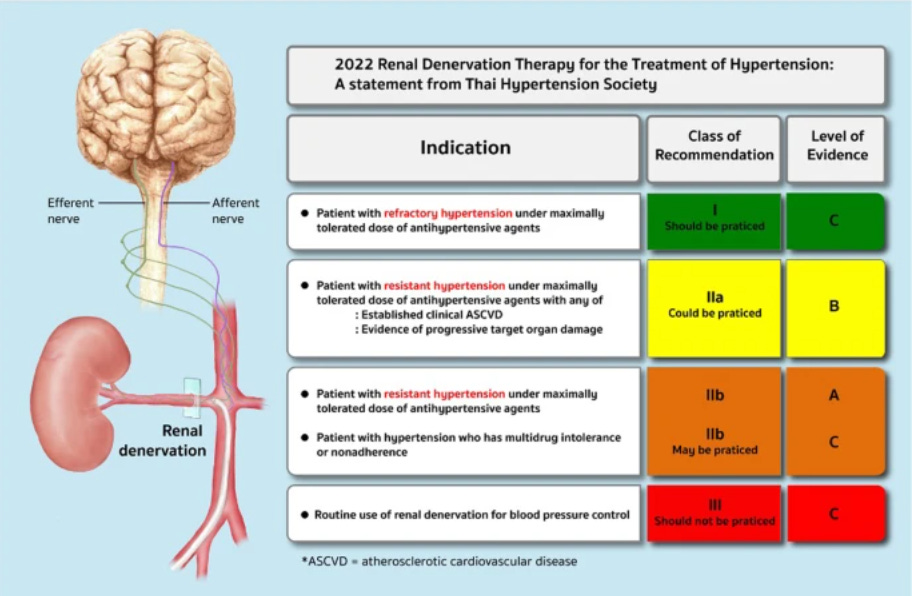
Schmieder et al, published a comprehensive review of the renal denervation (RDN) procedure with citations of the randomized trials. Reductions in systolic blood pressure of 5-10 mmHg have been shown in multiple trials with attenuation of treatment effect at six months.
My concern is loss of efficacy. The procedure appears to be generally safe when performed by expert operators. For patients with severe hypertension not controlled with multiple medications at risk for stroke, heart failure, or progressive loss of kidney function, the procedure should be discussed.
Please subscribe to Courageous Discourse as a paying or founder member so we can continue to bring you the truth.
Peter A. McCullough, MD, MPH
President, McCullough Foundation





I suspect that the covid vax has made a good portion of the population skeptical about new procedures, whether that skepticism is appropriate or not. Frankly, before I would even consider any of these new procedures cited, I would want to hear the opinions of the 2 that voted against Recor and the 7 that voted against Medtronic, and then do some due diligence. This covid fiasco, both in the way the disease was handled, as well as the vax, has, at least for me, made me extremely wary of medical opinions, agendas, procedures, etc.
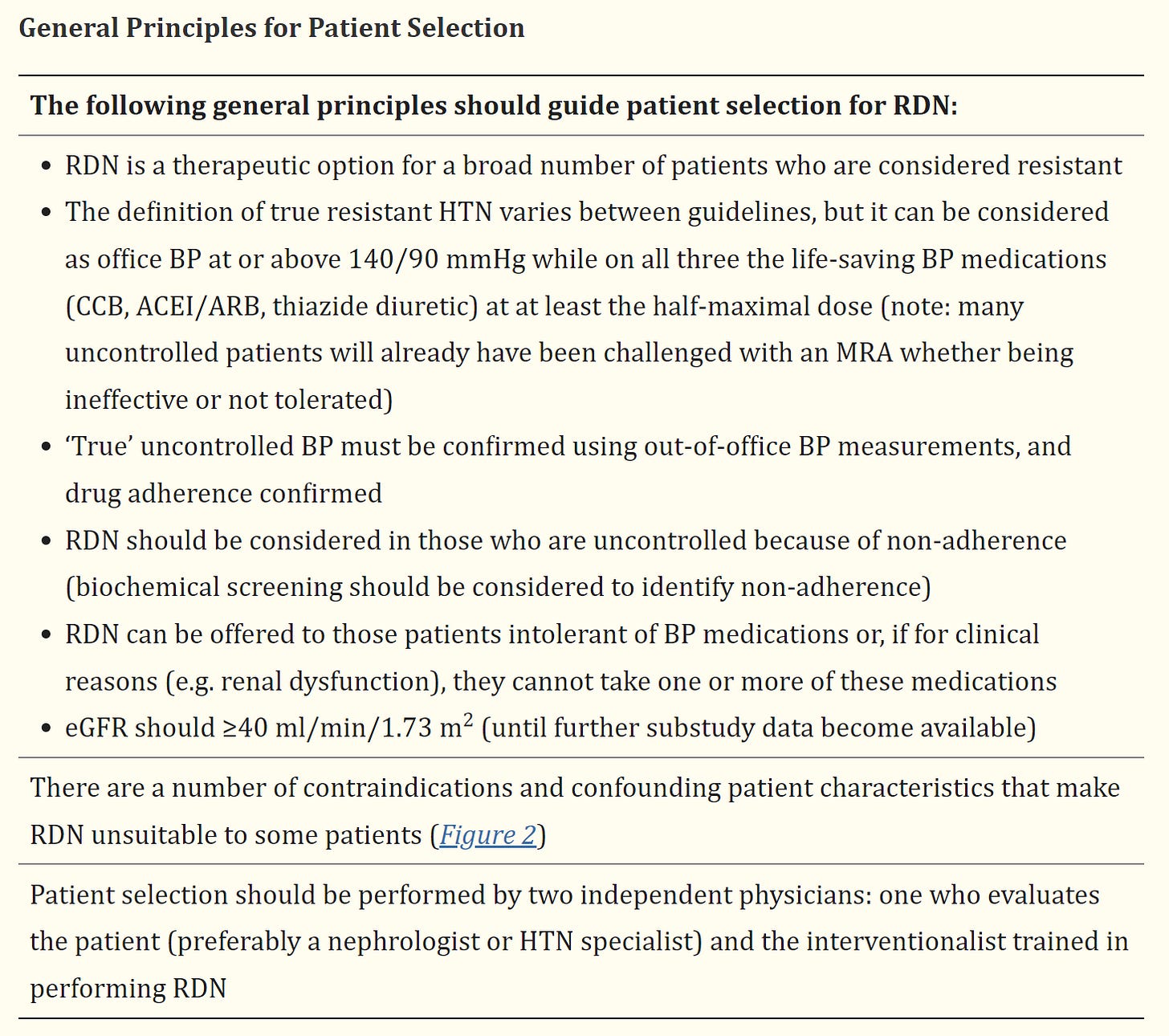
A BP of at or above 140/90 while on 3 different meds in different classes? I remember when HTN was defined a systolic BP of 140 and/or a diastolic BP of 90. Those numbers have been lowered in recent years. Now a sys BP of 130 or above or a diastolic of 80 or above is considered HTN. If you had a BP of 117/80 you would be considered to have HTN. I would think that a renal artery denervation procedure should be reserved for a higher cutoff point than AT or above 140/90 with all of the other selection criteria. Do the benefits really outweigh the risks at such a cutoff point?