RENOIR: Pfizer Trial Finds Adult RSV is Rare--Vaccine No Hospitalization or Death Benefit
Ambulatory RSV Occurred in <1% of all Subjects--Vaccine Not Worth the Effort in Adults
By Peter A. McCullough, MD, MPH
It is common for pharmaceutical companies to report companion trials in the same issue of the New England Journal of Medicine. The MATISSE and RENOIR trials were published together recently and our topic for this issue of Courageous Discourse is the RENOIR adult randomized placebo-controlled trial testing the Pfizer preFusion RSV antigen vaccine in adults. RSV is uncommon in adults and easily treated with nebulizers and advanced medications on occasion. As shown in this very large trial, RSV vaccination did not lead to reductions in hospitalization or adults in either the vaccine or placebo group—because no one become seriously ill.
RENOIR was stopped early at 34,284 participants had received RSVpreF vaccine (unadjuvanted RSVpreF vaccine at a dose of 120 μg (containing 60 μg each of RSV A and RSV B antigens) (17,215 participants) or placebo (17,069 participants). RSV-associated lower respiratory tract illness with at least two signs or symptoms occurred in 11 participants in the vaccine group and 33 participants in the placebo group, (vaccine efficacy, 66.7%; 96.66% confidence interval [CI], 28.8 to 85.8); 2 cases and 14 cases with least three signs or symptoms (vaccine efficacy, 85.7%; 96.66% CI, 32.0 to 98.7). RSV-associated acute respiratory illness occurred in 22 participants in the vaccine group and 58 participants in the placebo group, vaccine efficacy, 62.1%; 95% CI, 37.1 to 77.9). There were no hospitalizations or deaths mentioned in either group. Because data are sparse, we must rely on the lower limit of the confidence interval which is far below 50% for all outcomes, which is unacceptable.
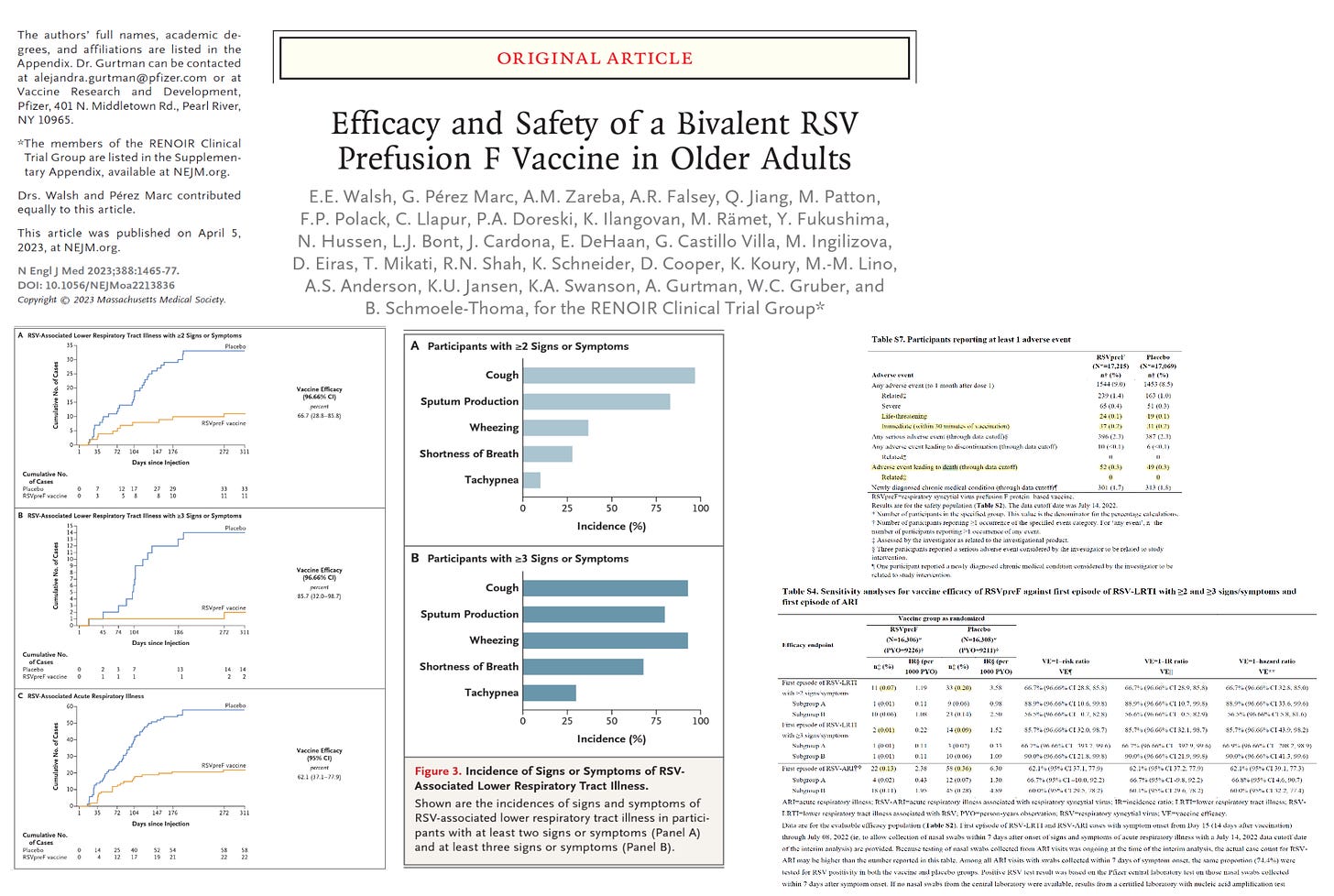
Safety of the vaccine was acceptable with the caveat there were more deaths in the vaccine group; none attributed to the vaccine by Pfizer (Supplemental Table 7).
As an adult medicine doctor, I can tell you this massive trial is unimpressive because RSV is so mild and rare in adults. In other words, 34,284 patients had to be randomized to spare 12 cases of RSV with three symptoms. So why bother with a short term vaccine when it can be easily treated with inhalers or nebulizers at home?
If you find “Courageous Discourse” enjoyable and useful to your endeavors, please subscribe as a paying or founder member to support our efforts in helping you engage in these discussions with family, friends, and your extended circles.





Are these trials named after impressionist painters because Pfizer wants to sound cultured and civilised, or is it because they just give the impression of success?
For once, accurate trial results. 😂 but yes, RSV has always been very rare in adults. Studied it about 15 years ago for a preemie and neonate vax, and that population was only moderately tenable at tens of thousands of dollars per vaccine. Now they are trying to RSV vax babies through their pregnant “birthing person”. Just say no. Too many ingredients in a cake mix tastes like sh1t after it’s baked.