Spirulina Supplementation for Hospitalized COVID-19 Patients
Safe, Natural, Positive Results
By Peter A. McCullough, MD, MPH
As we look back on the COVID-19 pandemic response debacle, a black spot is the lack of innovation or improved hospital care over time.
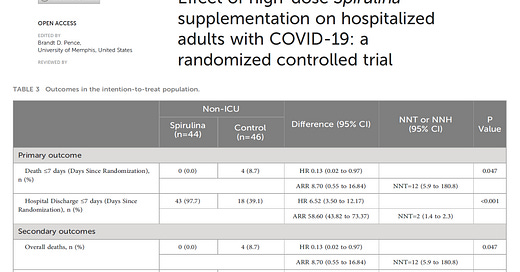
Aghasadeghi et al published a small randomized trial of oral supplementation with spirulina.
“Spirulina (arthrospira platensis), a filamentous, gram-negative cyanobacterium, is a blue-green microalga that is a non-nitrogen-fixing photoautotroph (19). Spirulina platensis is rich in protein (over 70%), vitamins, and minerals such as vitamin D, B12, provitamin A (beta carotene), and iron. It also contains phenolic acids, tocopherols, g-linolenic acid, and is particularly high in phycocyanobilin (PCB). PCB, a blue pigment protein, is part of the light-harvesting phycobiliprotein family and has anti-inflammatory, anti-cancer, and antioxidant properties. Spirulina platensis, which does not contain cellulose, is easily digestible (20–22). Previous studies have confirmed the anti-inflammatory properties of Spirulina platensis, demonstrating its ability to prevent and reduce inflammation by inhibiting histamine release from mast cells (20, 23, 24).”
To summarize, spirulina is a blue-green algae product. According to the National Institutes of Health (NIH), people have used doses of up to 19 g per day for a maximum of 2 months and up to 10 g per day for a maximum of 6 months. People should not exceed the dose stated on the product label—most products list a serving as 1 gram. Aghasadeghi described favorable directional changes in inflammatory markers and reductions in hard clinical outcomes with 15.2 g/day of spirulina supplementation in hospitalized COVID-19 patients.
Keep reading with a 7-day free trial
Subscribe to Courageous Discourse™ with Dr. Peter McCullough & John Leake to keep reading this post and get 7 days of free access to the full post archives.