Antiviral Monoclonal Antibodies Safe and Effective in Acute COVID-19
Clear Winner from Operation Warp Speed Goes Unrecognized by Public Health Officials and was Denied to Patients
By Peter A. McCullough, MD, MPH
I would have never thought five years ago that I would be using advanced monoclonal antibodies to treat acute viremia in a potentially fatal illness to help a patient avoid hospitalization or death. Yet, early research done by Dr. Ralph Baric who conceived SARS-CoV-2 in his 2015 papers also anticipated the development of a countermeasures such monoclonal antibodies directed against SARS-CoV-2.
Operation Warp Speed is an impressive public-private partnership to rapidly develop and test countermeasures against COVID-19. It’s main products were vaccines, oral antivirals, and monoclonal antibodies. COVID-19 vaccines, continue to be a debacle with record injuries, disabilities, and death with no proven reductions in hospitalization or death from proper trials. Sadly, Paxlovid and Mulnupiravir were very slow to develop and were not gamechangers in the pandemic.
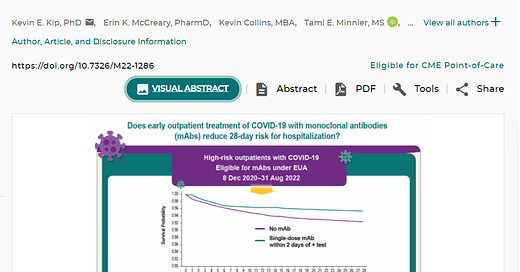
The clear winner from OWS was its very first acute ambulatory product, monoclonal antibodies. Kip et al used electronic medical records and comparative data for those treated and denied monoclonal antibodies for acute COVID-19. The risk for hospitalization or death at 28 days was 4.6% in 2571 treated patients and 7.6% in 5135 nontreated control patients (risk ratio [RR], 0.61 [95% CI, 0.50 to 0.74]). In subgroup analyses, those receiving monoclonal antibodies when the Alpha and Delta variants were presumed to be predominant had estimated RRs of 0.55 and 0.53, respectively, compared with 0.71 for the Omicron variant period. Relative risk estimates for individual products all suggested lower risk for hospitalization or death. Among immunocompromised patients, the RR was 0.45 (CI, 0.28 to 0.71).

Despite these impressive results, the US CMS has repeatedly pulled monoclonal antibodies off the market for predicted not actual losses in efficacy. Here is a list of CMS statements on the products:
“Bamlanivimab (EUA issued November 9, 2020, EUA revoked April 16, 2021). On April 16, 2021, the FDA revoked the EUA for bamlanivimab, when administered alone, due to a sustained increase in COVID-19 viral variants in the U.S. that are resistant to the solo product.
Bamlanivimab and etesevimab, administered together (EUA issued February 9, 2021, latest update January 24, 2022). On January 24, 2022, the FDA announced that due to the high frequency of the Omicron variant, bamlanivimab and etesevimab aren't currently authorized in any U.S. region. Therefore, you may not administer these drugs for treatment or post-exposure prevention of COVID-19 under the EUA until further notice.
Bebtelovimab (EUA issued February 11, 2022, latest update October 27, 2022). On November 30, 2022, the FDA announced that bebtelovimab isn’t currently authorized in any U.S. region because it isn’t expected to neutralize Omicron sub-variants BQ.1 and BQ.1.1. Therefore, you may not administer bebtelovimab for treatment of COVID-19 under the EUA until further notice.
REGEN-COV® (casirivimab and imdevimab, administered together) (EUA issued November 21, 2020, latest update January 24, 2022). On January 24, 2022, the FDA announced that due to the high frequency of the Omicron variant, REGEN-COV isn't currently authorized in any U.S. region. Therefore, you may not administer REGEN-COV for treatment or post-exposure prevention of COVID-19 under the EUA until further notice.
Sotrovimab (EUA issued May 26, 2021, latest update February 23, 2022). On April 5, 2022, the FDA announced that due to the high frequency of the Omicron BA.2 sub-variant, sotrovimab isn't currently authorized in any U.S. region. Therefore, you may not administer sotrovimab for treatment of COVID-19 under the EUA until further notice.
Update [1/26/2023] The U.S. Food and Drug Administration today revised the Emergency Use Authorization (EUA) for Evusheld (tixagevimab co-packaged with cilgavimab) to limit its use to when the combined frequency of non-susceptible SARS-CoV-2 variants nationally is less than or equal to 90%. Based on this revision, Evusheld is not currently authorized for use in the U.S. until further notice by the Agency.”
To summarize, US government agencies quickly pulled valuable life-saving treatments off the market for mutational changes in SARS-CoV-2 yet they continued to push obsolete COVID-19 vaccines. Monoclonal antibodies are high-tech and far more expensive than vaccines. Big pharma (Lilly, Regeneron, etc.) did not get a chance to see full utilization of their products. These observations suggest that the pandemic response was not all about money—it was about vaccine ideology. The Biopharmaceutical Complex was committed to a vaccine-only strategy and any products that offered hope for avoiding hospitalization and death were quickly taken away from suffering patients.
If you find “Courageous Discourse” enjoyable and useful to your endeavors, please subscribe as a paying or founder member to support our efforts in helping you engage in these discussions with family, friends, and your extended circles.
Hiding in Plain Sight The lab origin of SARS-CoV-2 was published in 2015.




I am not sure it is correct to say that the pandemic management was all about "vaccine ideology". This implies a belief in the vaccines as therapeutic agents. Although only peering into the minds of the corrupt public health officials and vaccine company leaders who directed the pandemic, such as Fauci, Birx, Collins, Becerra, Bancel, and Bourla is the only way of knowing, and we can't obviously do that, I believe that their intention was probably not to treat people, but to harm or kill people, and those who couldn't be disabled or murdered, would be sterilized, as part of a depopulation plan. Why else would they take all effective treatments off the market? Why would they approve only lethal treatments like remdesivir and intubation and assure that Dexamethasone could only be used at subtherapeutic doses. Why else would they recommend the vaccine for infants, children, and pregnant women, knowing it was causing massive harms and not stopping spread of the virus? The other goal I believe was probably in their minds, again unverifiable, is to get well over 90% of the population vaccinated so everyone could be put on vaccine passports. Vaccine passports would be a gateway to CBDC and to total surveillance and control over the surviving population. Although we can't fully assess the damages of the vaccine yet, the pandemic managers appear to have failed in their goals, if they were the ones I have said. Only about 70% of the population got the vaccine, if you can believe the data, and far fewer got the first booster. Even fewer got the second booster. Millions have been disabled and hundreds of thousands killed, but, according to the Rasmussen surveys, it appears that nearly half (and possibly more) of the population has become aware of the harmful nature of these vaccines. Their plan has undermined trust in the COVID-19 vaccine program and in the vaccine program in general. Now the globalists are trying to implement their plan without vaccine passports and with an ever increasingly distrustful population. They will try to foist their plan on us, but they are in for a serious fight. Let the best fighter win (we know who that will be).
“These observations suggest that the pandemic response was not all about money—it was about vaccine ideology.”
Or perhaps it was all about reducing the human population……considering that the drug companies, by virtue of their actual clinical trials, already knew the lack of efficacy and safety data with regard to the vaccine products, even before they were pushed out on to the public.