Chloroquine Highly Effective Treatment for Acute H5N1 Infection in Preclinical Model
Bird Flu Study in Human Cell and Mouse Models Confirms Therapeutic Success
By Peter A. McCullough, MD, MPH
Globally, from January 2003 to 31 March 2022, there have been 863 cases of human infection with avian influenza A(H5N1) virus reported from 18 countries. Of these 863 cases, 455 were fatal (CFR of 53%). Fatalities have occured in multiple countries. In the last twenty years, the nations with the most cases and deaths are Cambodia, China, Loas, and Viet Nam. However the casualty was reported from United Kingdom in January 2022.
Because highly pathogenic avian influenza is so infrequent, there have been no randomized trials or human studies of treatment. Therefore, we must rely on preclinical data with drugs that are already used and proven safe in humans.
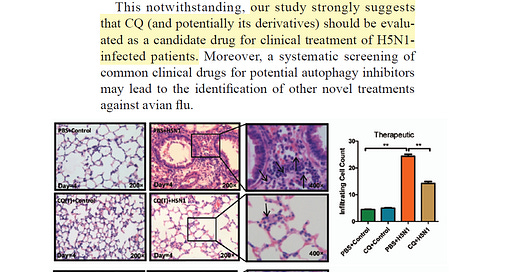
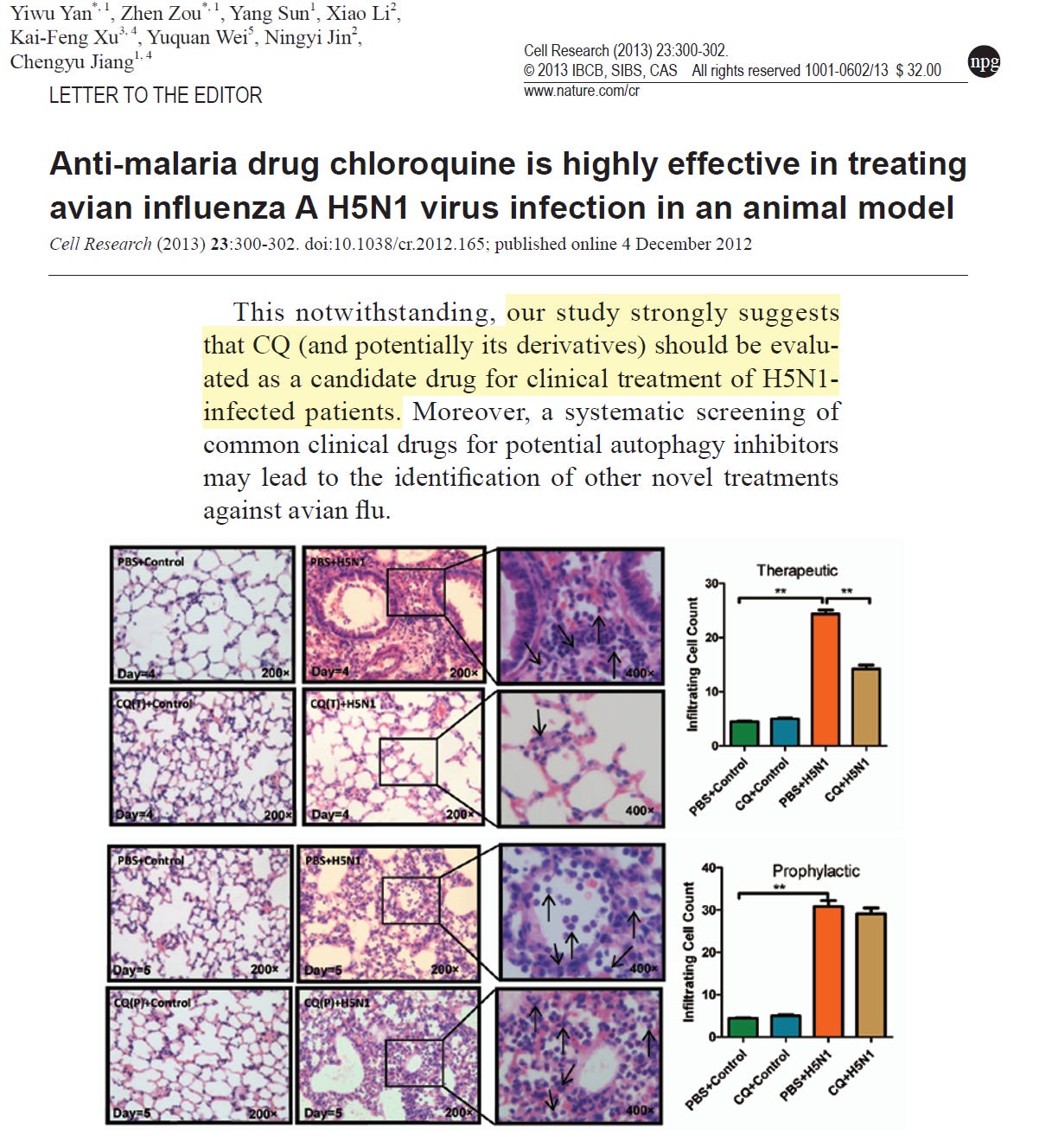
Yan, et al studied H5N1 infection in the laboratory and demonstrated that physiological relevant concentrations of chloroquine inhibited viral entry and damage to human cells. Additionally, when given as treatment and not prophylaxis, chloroquine reduced pulmonary alveolar infiltrates and improved survival in mice after a lethal dose of H5N1 from zero to 70%.
These data are encouraging if in the event we have more human cases among farm workers or if there is human to human spread, chloroquine or its derivative hydroxychloroquine would be reasonable therapeutic choices in the setting of high-risk or serious human avian influenza. Unfortunately, both chloroquine and hydroxychloroquine have toxicity and or expected ineffectiveness in livestock.
Please check out the black Contagion Kits at The Wellness Company. They contain hydroxychloroquine and have been extended to cover bird flu with oseltamivir phosphate.
Please subscribe to Courageous Discourse as a paying or founder member so we can continue to bring you the truth.
Peter A. McCullough, MD, MPH
Chief Scientific Officer, The Wellness Company





It’s disappointing that Dr. McCullough is touting Oseltamivir (Tamiflu). Oseltamivir (Tamiflu) never should have been approved by FDA or any other regulator. The drug trials were defined by the same kinds of problems that infect so many other highly profitable drugs peddled by the pharmaceutical industry. Dr. William Makis says pointedly “Tamiflu doesn’t work.” It doesn’t reduce hospitalization. It does cause many very serious adverse effects. It should not be in anybody’s pandemic preparedness kit.
Here’s Dr. Makis in a short video earlier this month explaining why Oseltamivir (Tamiflu) is dangerous and has no benefits. https://makismd.substack.com/p/new-podcast-15-minutes-with-drmakis-132. Among other sources, Dr. Makis cites a JAMA Systematic Review and Meta-Analysis published June 12, 2023 titled "Evaluation of Oseltamivir Used to Prevent Hospitalization in Outpatients With Influenza: A Systematic Review and Meta-Analysis,” https://pubmed.ncbi.nlm.nih.gov/37306992/. According to the JAMA article:
"Conclusions and relevance: In this systematic review and meta-analysis among influenza-infected outpatients, oseltamivir was not associated with a reduced risk of hospitalization but was associated with increased gastrointestinal adverse events. To justify continued use for this purpose, an adequately powered trial in a suitably high-risk population is justified."
The Indian Journal of Pharmacology recently elaborated on the many problems associated with Oseltamivir (Tamiflu) in an article suitably titled, “The Tamiflu Fiasco and Lessons Learnt” at https://journals.lww.com/iphr/fulltext/2015/47010/the_tamiflu_fiasco_and_lessons_learnt.3.aspx. The Indian Journal of Pharmacology relies in part on a Cochrane Review which also was highly critical of this dangerous, ineffective drug. Here are some excerpts about the dangers of Oseltamivir (Tamiflu) from the recent article:
"A recent Cochrane review and related articles have questioned the risk-benefit ratio of the drug, besides raising doubts about the regulatory decision of approving it.”
"Serious adverse events were first reported during post-marketing surveillance from Japan, UK, and subsequently from other places although most of the published articles did not report them. A recent Cochrane review and a series of articles in British Medical Journal (BMJ) have revealed the truth behind oseltamivir success story, which incidentally is one of the highest revenue earners for Roche."
"Post-marketing surveillance had uncovered adverse effects like raised liver enzymes, hepatitis, neuropsychiatric events, cardiac arrhythmia, skin hypersensitivity reactions including toxic epidermal necrolysis, Stevens-Johnson syndrome and erythema multiforme, metabolic side effects and renal events.[24] In some cases, increased QTc prolongation was seen in ECG in the treatment group compared with placebo during on-treatment periods. The most important serious adverse events which raised concerns were neuropsychiatric events such as depressed mood, behavior disturbance, panic attack, suicidal ideation, delusion, delirium, convulsion, and encephalitis."
"In other words, benefits had been overplayed, and harms had been underplayed in the reporting of the trials.[23]”
If scientist Dr. Mike Yeadon and data analyst Joel Smalley and others say there was no “pandemic”. No sarscov2. What is ivermectin and hcq effective against? Flu?