McCullough Protocol©: Nutraceuticals and Prescription Drugs
The Art and Science of Medicine Required to Face a Novel Threat
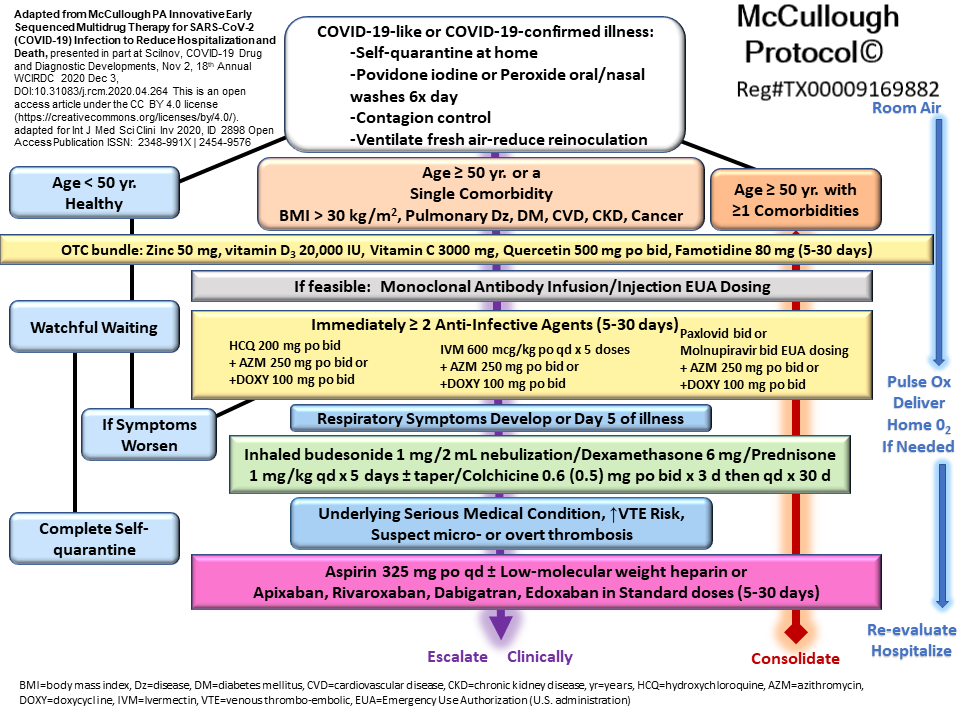
In this rapid review of the McCullough Protocol© during the inaugural week of “Courageous Discourse” we finish with the full protocol itself and have some focused comments on the use of nutraceuticals and prescription drugs specifically for the current Omicron after wave. The Wild-type through Delta waves of SARS-CoV-2 represented more severe and longer courses of illness among proportionally more initial COVID-19 infections or index cases. The current Omicron infection is characteristically mild with limited pulmonary and systemic involvement. For those who are having second and third infections it will be even more mild often indistinguishable from the common cold. All of the agents mentioned in this stack have reasonable supportive evidence. The therapeutic aims are to address viral replication, inflammation, and thrombosis. Patients at negligible and low risk can self-manage with viricidal nasal washes and the “OTC Bundle” which includes zinc, vitamin D, vitamin C, quercetin, famotidine (at four times the package dose—80 mg a day), and it’s reasonable to include aspirin 325 mg a day.[i] For very high-risk elderly patients with significant medical problems, for example, oxygen-dependent cardio-pulmonary disease monoclonal antibodies can be given as a one-time infusion preferably on days 1-3. In some regions of the U.S., bebtelovimab is available in emergency rooms and urgent care centers. If monoclonals are given, then oral antivirals can be omitted from the regimen. For those at moderate and above risk, it is reasonable to choose one oral antiviral (hydroxychloroquine, ivermectin, Paxlovid, molnupiravir) and consider combining it with an intracellular antibiotic (e.g., doxycycline or azithromycin) to cover concomitant atypical organisms and superimposed bacterial bronchitis. For fever control and inflammation, non-steroidal anti-inflammatories (e.g., naproxen) are favored over acetaminophen.[ii] In the Omicron wave, corticosteroids can be streamlined to inhaled budesonide and or oral prednisone. Beyond these principles the remainder of the McCullough Protocol© can be stylized to the patient and their particular history and symptoms with the goal of ameliorating the intensity and duration of symptoms and by that mechanism, reducing the risk of hospitalization or death. For example, patients with pleuropericardial symptoms or prior chest surgery, colchicine can be added and is well-supported by the largest outpatient placebo-controlled trial.[iii] If bedbound, immobile, or tendency for blood clotting, then anticoagulation with low-molecular weight heparin by injection or oral anticoagulants is prudent.
The community standard of care for COVID-19 was developed by doctors in the field who learned how to treat the infection using their clinical judgement and available sources of evidence—not by government agencies, state medical boards, or royal colleges of physicians. Having nearly three years of experience in ambulatory management, Dr. McCullough has learned from experts around the globe that no single drug is necessary nor sufficient to treat COVID-19. Yes, that means that the syndrome can be treated without antiviral agents as reported by Dr. Barrientos in El Salvador and Dr. Chetty in South Africa.[iv][v] Each protocol varies the intensity and classes of drugs based on style and phase of illness. As a general rule, day 1-3 is the golden window for initiation of early treatment, 4-6 agents are required, and the duration of therapy can be as short as 5 and as long as 30 days depending on the circumstances.[vi] Dr McCullough testified in the US Senate on January 24, 2022, that going forward 95% of all COVID-19 hospitalizations and deaths are avoidable with multidrug treatment.[vii] He emphasized in Texas Senate Testimony on June 27, 2022, that physicians have always had a duty to treat or refer ambulatory high-risk patients with COVID-19 so they could benefit from the “community standard of care.”[viii] Take a look at people in your circle who required hospitalization or even worse, died in the hospital—did they get the full McCullough Protocol© or an alternate regimen (FLCCC, ALFDS, Zelenko, Raoult, Barrientos, Chetty) during the days and weeks before admission? Were prehospital drugs continued via medication reconciliation once hospitalized? From the very first patient with the novel coronavirus to those falling ill at this time, outpatient physicians and mid-level providers are bolstered by Article 37 of the 2013 Declaration of Helsinki which essentially says that “unproven” interventions may be used after informed consent when in the doctor’s judgment it “offers hope of saving life, re-establishing health or alleviating suffering.” Conclusive randomized, blinded, placebo-controlled trials of outpatient multidrug regimens are not forthcoming. Such trials would require sample sizes of 20,000 to 40,000 patients. Rather, ambulatory treatment of COVID-19 has evolved relying on clinical judgement and the use of drugs with a signal of benefit and acceptable safety in line with the 21st Century Cures Act.[ix] One of the greatest crimes of all-time has been the systematic suppression and oblivion to early therapy over course of the crisis. We explore how therapeutic nihilism swept across the globe to cause catastrophic harm in our book “Courage to Face COVID-19: Preventing Hospitalizations and Deaths while Battling the Biopharmaceutical Complex.”[x]
[i] Mura, C., Preissner, S., Nahles, S. et al. Real-world evidence for improved outcomes with histamine antagonists and aspirin in 22,560 COVID-19 patients. Sig Transduct Target Ther 6, 267 (2021). https://doi.org/10.1038/s41392-021-00689-y
[ii] Fazio S, Bellavite P, Zanolin E, McCullough PA, Pandolfi S, Affuso F. Retrospective Study of Outcomes and Hospitalization Rates of Patients in Italy with a Confirmed Diagnosis of Early COVID-19 and Treated at Home Within 3 Days or After 3 Days of Symptom Onset with Prescribed and Non-Prescribed Treatments Between November 2020 and August 2021. Med Sci Monit. 2021 Dec 30;27:e935379. doi: 10.12659/MSM.935379. PMID: 34966165; PMCID: PMC8725339.
[iii] Tardif JC, Bouabdallaoui N, L'Allier PL, Gaudet D, Shah B, Pillinger MH, Lopez-Sendon J, da Luz P, Verret L, Audet S, Dupuis J, Denault A, Pelletier M, Tessier PA, Samson S, Fortin D, Tardif JD, Busseuil D, Goulet E, Lacoste C, Dubois A, Joshi AY, Waters DD, Hsue P, Lepor NE, Lesage F, Sainturet N, Roy-Clavel E, Bassevitch Z, Orfanos A, Stamatescu G, Grégoire JC, Busque L, Lavallée C, Hétu PO, Paquette JS, Deftereos SG, Levesque S, Cossette M, Nozza A, Chabot-Blanchet M, Dubé MP, Guertin MC, Boivin G; COLCORONA Investigators. Colchicine for community-treated patients with COVID-19 (COLCORONA): a phase 3, randomised, double-blinded, adaptive, placebo-controlled, multicentre trial. Lancet Respir Med. 2021 Aug;9(8):924-932. doi: 10.1016/S2213-2600(21)00222-8. Epub 2021 May 27. PMID: 34051877; PMCID: PMC8159193.
[iv] Three Years, Hundreds of Millions of Patients, So Many Ways to Treat by Dr. Peter McCullough | Oct 1, 2022 | Health, Politics
[v] Evidence-Based Medicine Supports the Practice of the Art of Medicine by Dr. Peter McCullough | Jul 4, 2022 | Health, Politics,
[vi] McCullough PA, Alexander PE, Armstrong R, Arvinte C, Bain AF, Bartlett RP, Berkowitz RL, Berry AC, Borody TJ, Brewer JH, Brufsky AM, Clarke T, Derwand R, Eck A, Eck J, Eisner RA, Fareed GC, Farella A, Fonseca SNS, Geyer CE Jr, Gonnering RS, Graves KE, Gross KBV, Hazan S, Held KS, Hight HT, Immanuel S, Jacobs MM, Ladapo JA, Lee LH, Littell J, Lozano I, Mangat HS, Marble B, McKinnon JE, Merritt LD, Orient JM, Oskoui R, Pompan DC, Procter BC, Prodromos C, Rajter JC, Rajter JJ, Ram CVS, Rios SS, Risch HA, Robb MJA, Rutherford M, Scholz M, Singleton MM, Tumlin JA, Tyson BM, Urso RG, Victory K, Vliet EL, Wax CM, Wolkoff AG, Wooll V, Zelenko V. Multifaceted highly targeted sequential multidrug treatment of early ambulatory high-risk SARS-CoV-2 infection (COVID-19). Rev Cardiovasc Med. 2020 Dec 30;21(4):517-530. doi: 10.31083/j.rcm.2020.04.264. PMID: 33387997.
[vii] Dr. Peter McCullough Full Highlights | Senator Ron Johnson COVID-19: A Second Opinion, January 24, 2022
[viii] Dr. Peter McCullough, MD, MPH, Jun 27, 2022 Texas Senate HHS Testimony
[ix] Public Law 114–255 114th Congress An Act To accelerate the discovery, development, and delivery of 21st century cures, and for other purposes. December 13, 2016
[x] Leake J, McCullough PA. THE COURAGE TO FACE COVID-19: Preventing Hospitalization and Death While Battling the Bio-Pharmaceutical Complex





Dear Doctor McCullough,
I have concerns regarding the safety profile of the Merck antiviral product, molnupiravir. As I've written in "#MERCKyBusiness - IS "THE CURE" WORSE THAN THE DISEASE?", the mutagenic investigation that was conducted by Merck was extremely problematic.
For example, the pig-a method, unlike alkaline comet assay, does not detect pre-mutagenic DNA lesions. Also, Pig-a gene mutation in RBCs seems to be less accurate than measuring gene mutations in reticulocytes (RET) (immune red blood cells).
Similarly, the transgenic rodent mutation (TGR) assay used to show the safety is suitable for the detection of point mutations, insertions and small deletions but not large deletions, and It does not seems to detect pre-mutagenic lesions.
There are more concerns that I've raised. Please see all the details and the links to the relevant reference in my post on the topic:
https://ehden.substack.com/p/merckybusiness
I would love to hear your comment on my observations and concerns.
Kind Regards,
Ehden Biber
Paxlovid needs to be taken off the protocol;. Big time problems with it, Dr. McCullough.