Pfizer MATISSE Trial Targets Pregnant Women for Bivalent RSV Vaccine
Unprecedented Pregnancy Study Fails on Efficacy, Durability, and Offers No Assurances on Long-Term Safety
Peter A. McCullough, MD, MPH
Vaccine ideology or hubris appears everywhere! Never had we heard so much about vaccines in our day to day life as laypersons or as healthcare professionals. This certainly is the case for respiratory syncytial virus (RSV) which is largely an infantile problem easily treated with nebulizers when it occurs. Now Pfizer is aggressively testing their investigational bivalent RSV prefusion protein vaccine which contains stabilized preF glycoproteins from the two main cocirculating antigenic subgroups (RSV A and RSV B). Is this really needed? Should pregnant women be put at risk even before product launch for the general childhood population? We can see now that emboldened Pfizer is breaking new ground with clinical trials in late term pregnancy.
The MATISSE Study group conducted a phase 3, double-blind trial where they randomly assigned, in a 1:1 ratio, pregnant women at 24 through 36 weeks’ gestation to receive a single intramuscular injection of 120 μg of a bivalent RSV prefusion F protein–based (RSVpreF) vaccine or placebo. The main primary efficacy end point was rare and occurred in <2% at all time points and was defined as medically attended severe RSV-associated lower respiratory tract illness in the babies within 90, 120, 150, and 180 days after birth. A lower boundary of the confidence interval for vaccine efficacy (99.5% confidence interval [CI] at 90 days; 97.58% CI at later intervals) greater than 20% was considered to meet the success criterion for vaccine efficacy with respect to the primary end point. This is far lower than a reasonable acceptance limit of 50% for the lower bound. As you can see at all times, the real effect size could have been lower than 50%. When outcomes are sparse, the point of central tendency is a statistical blur, hence we must rely on the bounds of the confidence limits. Additionally, protection waned rapidly after birth and was not demonstrated to last 12 months.
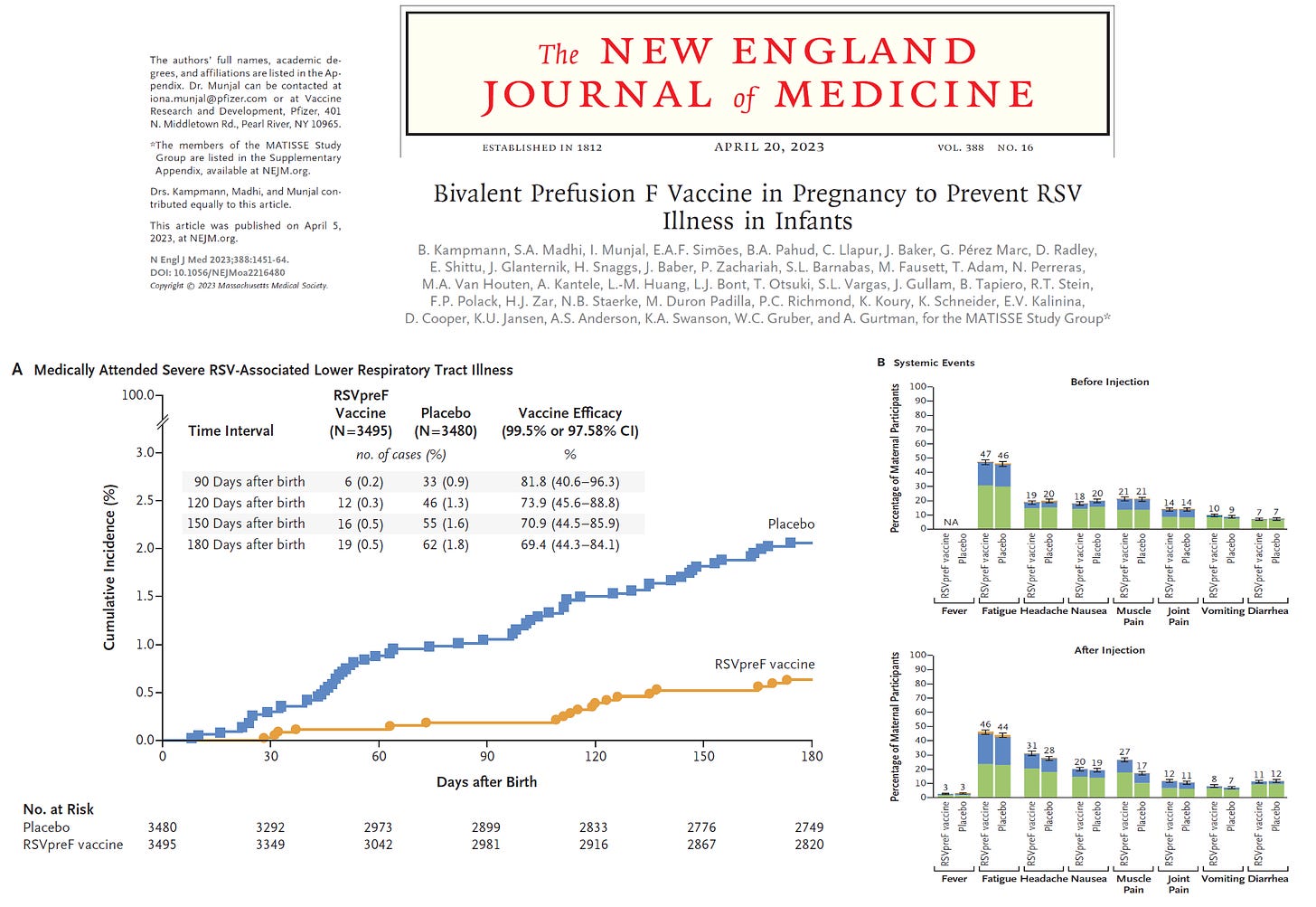
In summary, Pfizer has aggressively advanced RCTs into the pregnant population with no assurances on long term outcomes. There is no direct benefit to the mothers. Furthermore, the sponsors moved the goal posts to make it easer to have a successful trial. We should demand long-term safety, high efficacy (>50% VE as lower bound of CI), and at least one year of durability, for such a rare and easy-to-treat condition in babies.
If you find “Courageous Discourse” enjoyable and useful to your endeavors, please subscribe as a paying or founder member to support our efforts in helping you engage in these discussions with family, friends, and your extended circles.





These people (?) are monsters. Why we permit them to exist and prey upon us is beyond me. The sooner collapse hits the US, the better for Humankind. We are infested with and controlled by clinically certifiable psychopaths at all levels - with a demented hair sniffer as captain of the ship.
OMG! I cannot believe these people persist on doing this. The issue of vaccinating pregnant women for diseases of negligible importance should be one of ethical principles and not scientific considerations. It is unacceptable to intentionally expose a pregnant woman to a potentially harmful intervention as an "experiment". This study should not have been approved by an IRB. Then, after the fact, the results are so bad. They insist on reporting RRR, which was bad anyway. The ARR was negligible; out of > 3,500 participants per group, only 24 and 56 subjects had the "outcome of interest". The shame, as usual, is that authors continue to conclude that their results are so good... in order to have their articles published. It is disgusting.