BREAKING - New Study Demands Immediate Moratorium on COVID-19 mRNA Injections
Pfizer/BioNTech’s COVID-19 modRNA Vaccines: Dangerous Genetic Mechanism of Action Released before Sufficient Preclinical Testing
By Nicolas Hulscher, MPH
As calls for an immediate moratorium on COVID-19 mRNA injections intensify…
…Oldfield et al just published a new study in the Journal of American Physicians and Surgeons titled, Pfizer/BioNTech’s COVID-19 modRNA Vaccines: Dangerous Genetic Mechanism of Action Released before Sufficient Preclinical Testing:
Here’s a concise summary of the study’s main points:
Regulatory Classification and Guidelines:
COVID-19 modRNA vaccines were misclassified as traditional vaccines rather than gene therapy products, bypassing stricter regulatory requirements.
WHO guidelines from 2005 were used for nonclinical assessments, despite being outdated and inapplicable to modRNA vaccines.
Spike Protein and Safety Concerns:
The spike protein produced by modRNA vaccines is toxic and can cause immune-related complications.
Studies on pharmacokinetics (how much spike protein is produced, where it distributes, and how long it persists) were not conducted as part of Pfizer’s submission.
Spike protein production varies among individuals based on genetics, health status, and other factors, but this variability was never studied.
Biodistribution and LNP Behavior:
Pfizer’s own biodistribution data showed lipid nanoparticles (LNPs) and modRNA were widely distributed throughout the body, including the liver, spleen, ovaries, and adrenal glands.
Contrary to claims, spike protein production was not limited to the injection site, raising concerns about off-target effects and toxicity.
Toxicology and Carcinogenicity Testing:
Pfizer did not conduct genotoxicity or carcinogenicity studies, despite the potential for the spike protein and LNPs to cause harm.
Wistar Han™ rats, which are not a relevant species for SARS-CoV-2 toxicity studies, were used inappropriately in preclinical testing.
Deficient Clinical Trials:
Phase I clinical trials failed to measure critical factors like the amount and variability of spike protein produced in individuals.
Placebo groups in initial trials were unblinded early, undermining the integrity of long-term safety and efficacy data.
Adverse Events and VAERS Reporting:
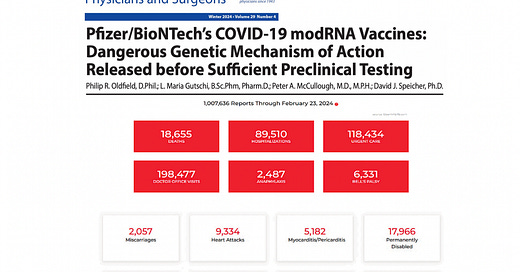
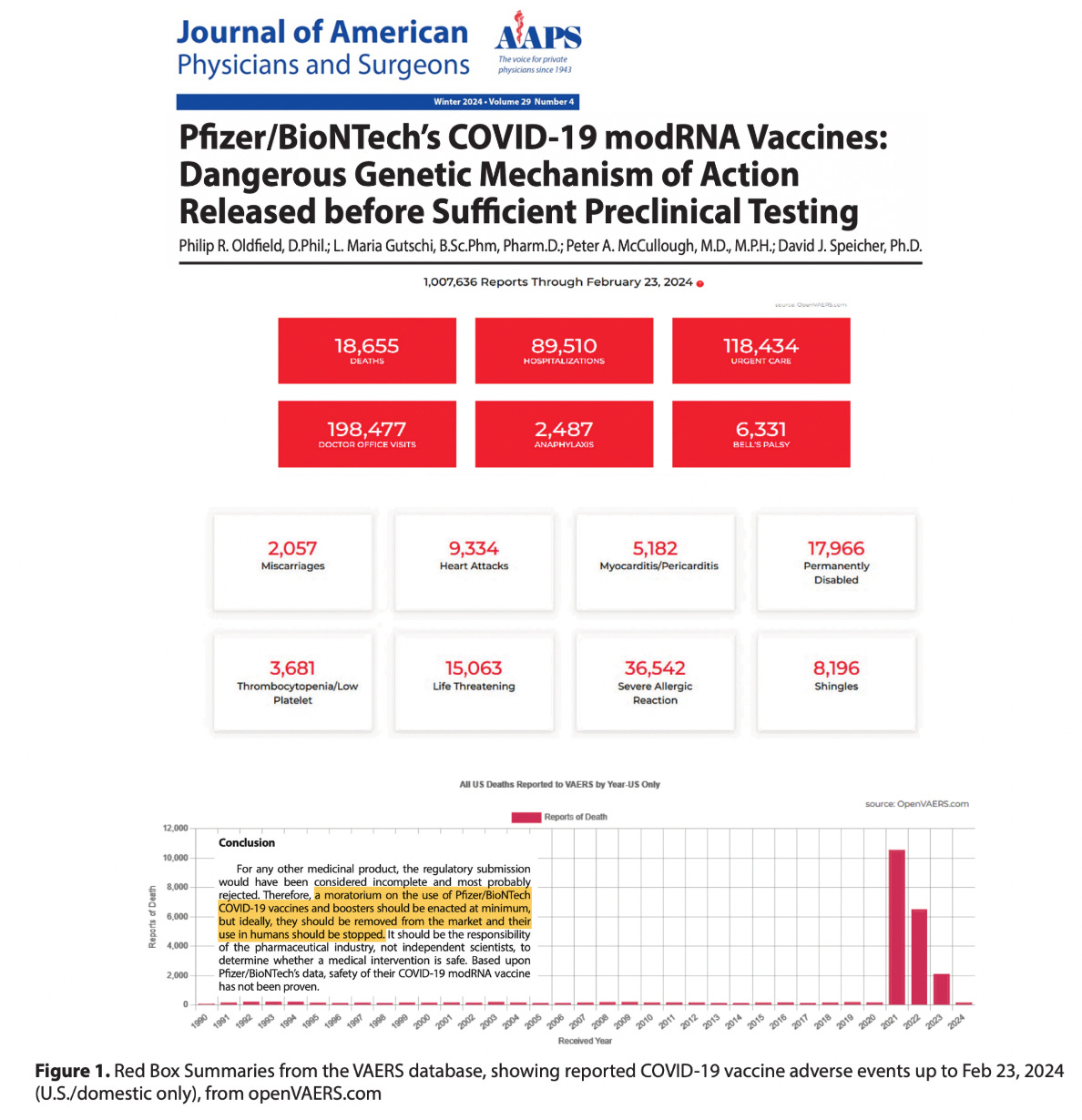
Post-authorization data from Pfizer showed 1,223 deaths and numerous serious adverse events within the first three months of vaccine rollout.
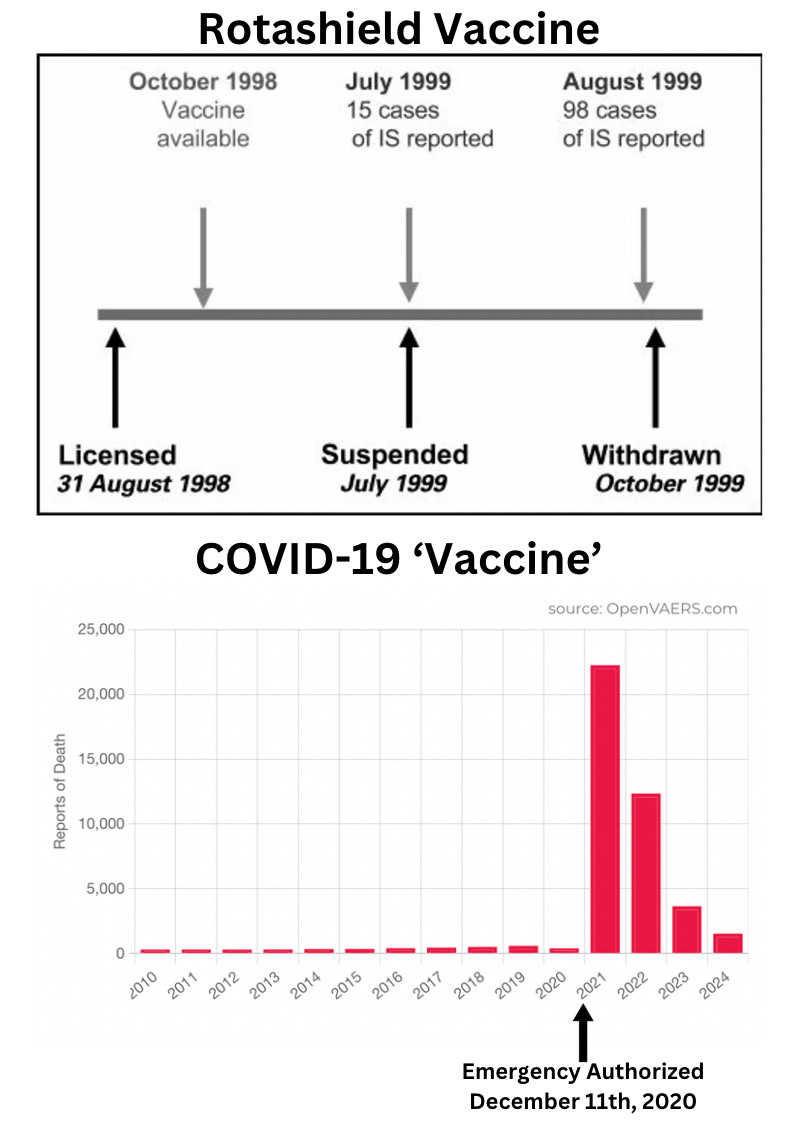
The Vaccine Adverse Event Reporting System (VAERS) documented a huge spike in reported adverse events following the COVID-19 vaccine rollout, far exceeding reports for previous vaccines.
Despite the massive increase in reported deaths and injuries, no corrective actions were taken. This contrasts with earlier vaccines like Rotashield®, which were removed after only 15 cases of intussusception:
Regulatory Oversight Failures:
The FDA and other global regulatory agencies overlooked or dismissed major concerns raised during the review process.
Regulatory assessments relied on insufficient data and assumptions rather than rigorous scientific evidence.
Call for a Moratorium:
Based on incomplete data, serious safety concerns, and potential long-term risks, the authors recommend a moratorium on Pfizer/BioNTech’s COVID-19 modRNA vaccines.
Proper studies must be conducted to ensure safety, as the responsibility lies with the pharmaceutical industry, not independent scientists.
Since the proper conditions for market withdrawal have been met, the primary obstacle preventing an immediate moratorium on COVID-19 genetic injections is our captured regulatory agencies, which will hopefully be dealt with after the Senate confirmation process.
Nicolas Hulscher, MPH
Epidemiologist and Foundation Administrator, McCullough Foundation
www.mcculloughfnd.org
Please consider following the McCullough Foundation and Nicolas Hulscher on X (formerly Twitter) for further content.