By Peter A. McCullough, MD, MPH
In epidemiology, external validity or generalizability is very important. This means that when a finding is discovered in one population, that it is confirmed in another with the same exposure. I was fortunate to interview Dr. Vibeke Manniche, PhD, a Danish epidemiologist who is the lead author an a recent publication comparing suspected adverse events after injection with Pfizer-BioNTech BNT162b2 COVID-19 COMIRNATY® vaccine in Sweden and Denmark.
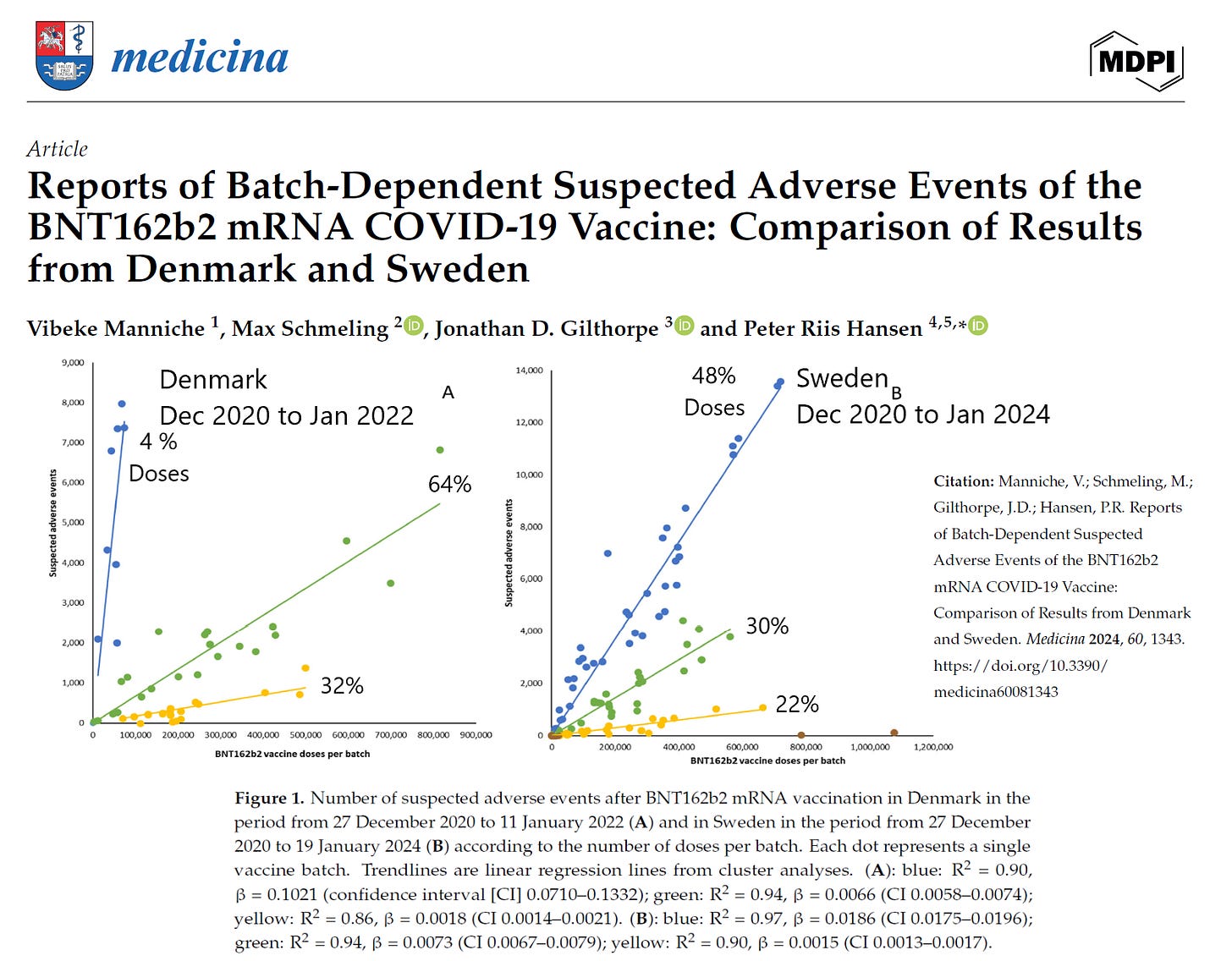
The major findings are:
Denmark highest risk batches of vaccines released December 2020 to March 2021, in Sweden the high risk batches continued to June, 2021
The proportion of low risk batches where essentially nothing happens after injection was 32% in Denmark and 22% in Sweden. This is good news for the 75% of Americans who took the shot—up to a third may be in the clear.
The blue high risk batches (12 overlapped with Denmark) was a larger group in Sweden and comprised 48% of the doses, much larger than the 4% in Denmark.
Please listen to the entire interview and read the manuscript from Manniche et al. She is suspicious that Pfizer knew serious events including death were occuring with the early formulations and they must have made a change, possibility reducing the quantity of mRNA to lower the risks of later batches. These findings are important for patients and clinicians to record the vaccine history and when the shots were taken, and then look up their Pfizer batch to understand the risks of what was injected into their bodies. Many batches have had zero serious side effects and one can only hope they have by good fortune received their doses from those benign batches. These data apply to Pfizer only. TrialSite News reviewed this paper and stated that batch heterogeneity should have called for immediate safety reviews by public health authorities, yet nothing happened. The world deserves full analysis of all the vaccines by batch and information back to recipients about the adverse events that have occured with doses given out of their vials.
Please subscribe to Courageous Discourse as a paying ($5 monthly) or founder member so we can continue to bring you the truth.
Peter A. McCullough, MD, MPH
President, McCullough Foundation














Share this post